A car battery doesn't always explode just when beingjump-started or charged. There are cases where they suddenlyexplode due to the installed position, for example, or asa result of a crash, as has been alleged in the probe leading tothe Chevy Volt recall.
|In any case, automotive battery explosions can cause severeinjury and can even be fatal, depending on where one isstanding during the explosion. These explosions present addedconsiderations for adjusters who are tasked not only withprocessing the resultant claims but also in identifyingsubrogation opportunities.
|Let's first examine the anatomy of a typical automotive batterybefore delving into cases of catastrophic failure. As shown inFigure 1 below an automotive battery, typically oflead/acid construction, is an electrochemical container thatproduces voltage, which causes electrical current to flow tovarious components in an automotive vehicle. An outer polymer case(high density polypropylene) acts as a container for an electrolyte(sulfuric acid), six cells and lead plates. Each cell delivers 2.1volts, with a total voltage of 12.6 volts, at fullcharge. Vents are installed at the top of the battery to ventgasses formed during the normal charging cycles.
|
 During the charging cycle, hydrogen gas is generated andaccumulates in the head space above the electrolyte level, prior toventing. Hydrogen gas has a wide range of explosive limits in air,ranging from 4 to 72-percent hydrogen in air and is easily ignitedby a flame or spark. If the hydrogen is ignited inside the battery,then it typically blows off the top of the battery case, showeringsulfuric acid in the immediate vicinity along with fragments of thebattery case. The explosive energy generation is so rapid that thevents cannot relieve the pressure in time to prevent anexplosion.
During the charging cycle, hydrogen gas is generated andaccumulates in the head space above the electrolyte level, prior toventing. Hydrogen gas has a wide range of explosive limits in air,ranging from 4 to 72-percent hydrogen in air and is easily ignitedby a flame or spark. If the hydrogen is ignited inside the battery,then it typically blows off the top of the battery case, showeringsulfuric acid in the immediate vicinity along with fragments of thebattery case. The explosive energy generation is so rapid that thevents cannot relieve the pressure in time to prevent anexplosion.
Figure 2 to the upper right is a view of atwo-year-old battery that exploded, causing personal injury fromacid burns. This occurred when a standby generator was startingduring its normal maintenance test cycle. The top of the batterywas blown away, suggesting that hydrogen was ignited inside thebattery.
|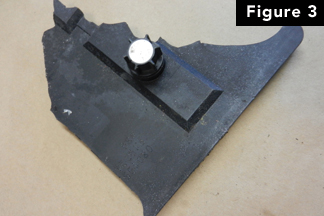
 Figure 3 here shows a batteryfragment that was found imbedded in the ceiling of the buildingthat enclosed the generator. Inspection of the battery shown inFigure 2 revealed that one of the intercellconnectors (Figure 4) was loose and corroded.
Figure 3 here shows a batteryfragment that was found imbedded in the ceiling of the buildingthat enclosed the generator. Inspection of the battery shown inFigure 2 revealed that one of the intercellconnectors (Figure 4) was loose and corroded.
A loose connection inside a battery can result in an electricalarc jumping across the gap, igniting the hydrogen. In thiscase, the loose connection was determined to be a result of amanufacturing defect.
||Other internal explosive ignition conditions may exist that arenot related to a manufacturing defect:
- A conductive bridge may be formed between two plates as aresult of low electrolyte levels. When a high current demand isplaced on the battery, it can arc, igniting hydrogen gas andinitiating an explosion. This is a result of improper batterymaintenance where the electrolyte level should be monitoredperiodically and lost electrolyte replaced. Maintenance freebatteries have a hydrometer that measures the specific gravity ofthe electrolyte (an indicator of the concentration of theelectrolyte and hence, the electrolyte level) and indicates whetheror not a battery should be replaced.

- External ignition sources often manifest themselves in the formof loose battery cable connections or a poor connection withbattery charger clamps that generate an electrical arc. Jumpstarting vehicles with dead batteries can result in electrical arcsat the dead battery terminals if the last set of clamps is attachedto the dead battery. Recommended procedure is to attach the jumperclamps to the dead battery first and then to the live battery.
- Battery explosions have occurred as a result of tools beingplaced between the battery terminals. Some individuals test abattery by placing a screw driver across the terminals to see if anarc jumps, revealing whether the battery is supplying electricalenergy or not. This can result in a battery explosion since thecurrent through the screw driver is not regulated, can be very highand generate an electrical arc, causing an internal or externalexplosion.
- Corrosion, which can cause electrical resistance and a possiblearc ignition source, may develop at battery terminals as shown inFigure 5, which also illustrates a loose orimproperly secured battery clamp.
As in most investigations, retention of the evidence isdesirable if subrogation is anticipated. In battery explosionlosses, fragments may be found scattered throughout the scene andimbedded in the building structure. Collecting these pieces helpsthe technical analyst determine the cause of the explosion. It isencouraged to have the battery analyzed in a timely manner toreduce the effect of corrosion, which can obscureevidence.
Want to continue reading?
Become a Free PropertyCasualty360 Digital Reader
Your access to unlimited PropertyCasualty360 content isn’t changing.
Once you are an ALM digital member, you’ll receive:
- All PropertyCasualty360.com news coverage, best practices, and in-depth analysis.
- Educational webcasts, resources from industry leaders, and informative newsletters.
- Other award-winning websites including BenefitsPRO.com and ThinkAdvisor.com.
Already have an account? Sign In
© 2024 ALM Global, LLC, All Rights Reserved. Request academic re-use from www.copyright.com. All other uses, submit a request to [email protected]. For more information visit Asset & Logo Licensing.








